You have 0 items in your cart
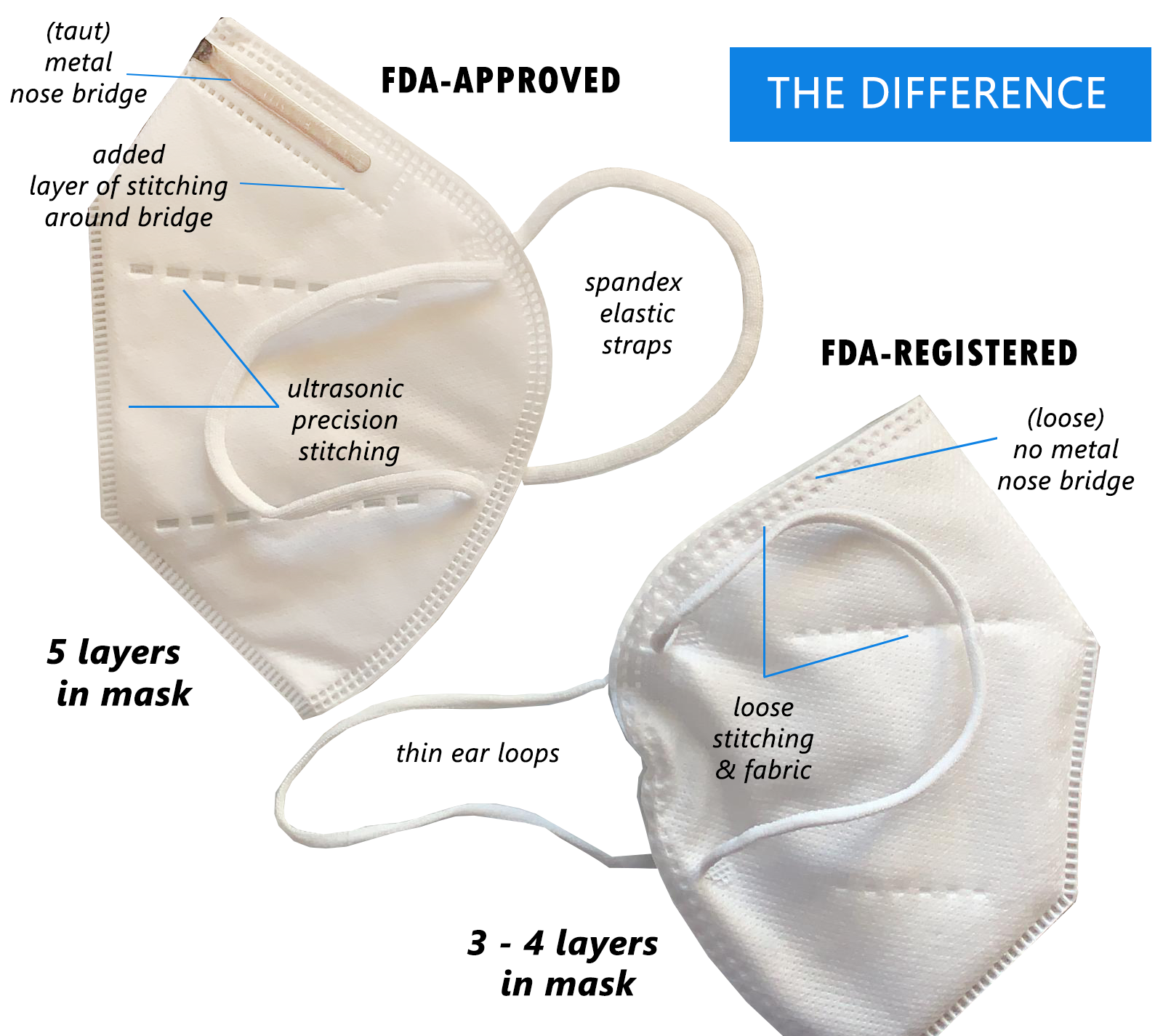
FDA-Approval in the Works
On April 3, 2020, the FDA issued an EUA (emergency use authorization) letter announcing that the agency will authorize the KN95 masks for use in healthcare settings if it meets certain criteria.
We are currently working with the FDA to be added to Appendix A as an authorized manufacturer/importer of KN95 respirators.
You can find the original article here – https://www.fda.gov/media/136664/download
Additionally, you can find a repository of all PPE letters of authorization at the link below: